Restoring mobility to Life
LytoJoint™

Regenerating the life in Motion

LytoJoint™ is a proven offering of Lytone Enterprise, Inc.
Holding patents across Taiwan, China, USA, and Japan.
Safe
LytoJoint™ is a safe, research validated. Ingredients sourced only from Taiwan and Japan.
Anti-Inflammation
The anti-inflammatory benefits of LytoJoint™ come from it’s regenerative formula.
Trademark
LytoJoint™ is a registered trademark of Lytone Enterprise, Inc. Ingredients sourced only from Taiwan and Japan.
Patented
LytoJoint™ is a worldwide patented and proprietary nutraceutical product and is a registered trademark of Lytone Enterprise, Inc.
Features of LytoJoint™
Animal Experiment Data of LytoJoint™ from the Food Industry Research and Development Institute
Features of LytoJoint™
Animal Experiment Data of LytoJoint™ from the Food Industry Research and Development Institute
01.
Relieve Arthritis Pain
Significant pain relief for arthritis
02.
Support Joint Recovery
Repairs damage caused by arthritis
03.
Strengthen Your Bones
Increase in bone mineral density and trabecular quantity
04.
Improve Bone Strength
An increase in trabecular thickness
01.
Relieve Arthritis Pain
Significant pain relief for arthritis
02.
Support Joint Recovery
Repairs damage caused by arthritis
03.
Strengthen Your Bones
Increase in bone mineral density and trabecular quantity
04.
Improve Bone Strength
An increase in trabecular thickness
| Joint Ingredients / Efficacy |
Restore cartilage | Anti-inflammatory | Nutrition (bone & cartilage interface) |
Pain relief |
|---|---|---|---|---|
| LytoJoint™ | ||||
| Glucosamine-HCL, Chondroitin, Hyaluronic acid, N-acetylglucosamin |
||||
| Type II collagen (un-denatured form) |
||||
| Type II collagen (denatured form) |
||||
| Herbal Extract (Curcumin) |
How Bad is Osteoarthritis?
>3 billion people diagnosed worldwide.
50% of patients are under
65 years old.
Prevalence increases sharply after age 40
No effective western medical treatment before joint replacement surgery.
A leading cause of disability worldwide
Average direct medical cost per OA patient:
10,000-25,000 USD/year
Osteoarthritis Therapeutics Market Size and Forecast 2024 to 2034 (USD Billion)
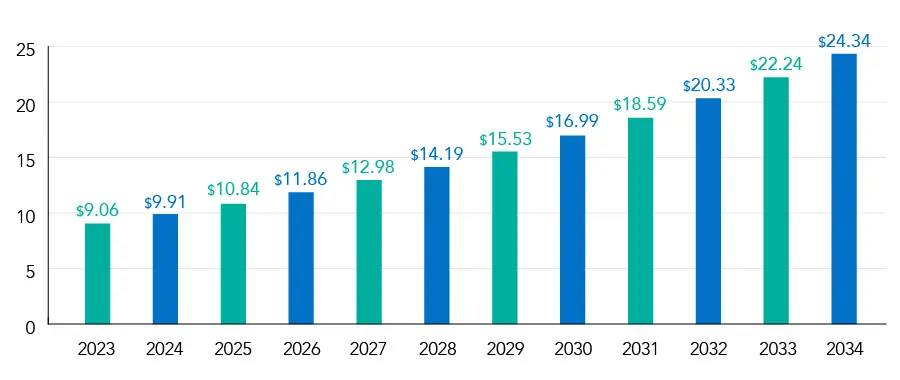
Source: Precedence Research
Asia-Pacific is predicted to have a Compound Annual Growth Rate (CAGR) of 8.7%
The European osteoarthritis therapeutics market value was USD 4.66 billion in 2024 and is projected to be worth around USD 11.44 billion by 2034, growing at a CAGR of 9.86% from 2024 to 2034.
Osteoarthritis Therapeutics Market Share, By Region, 2023 (%)
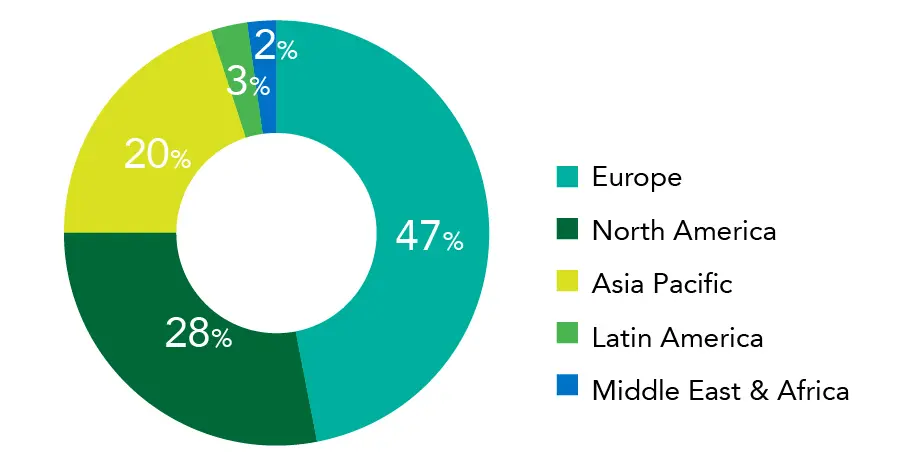
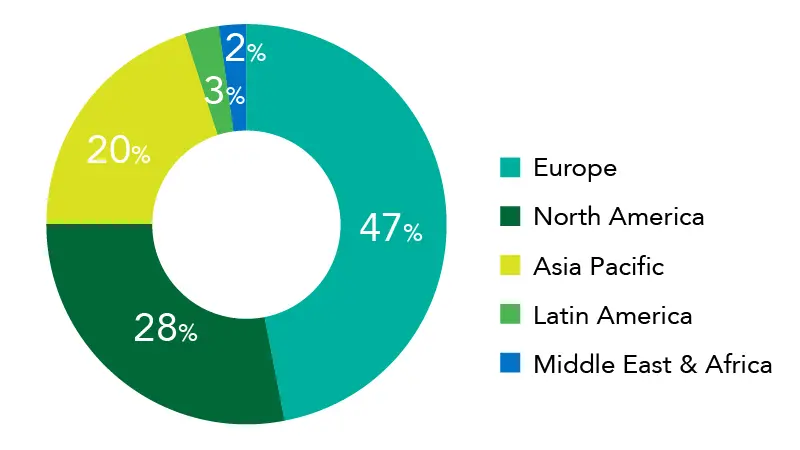
Source: Precedence Research
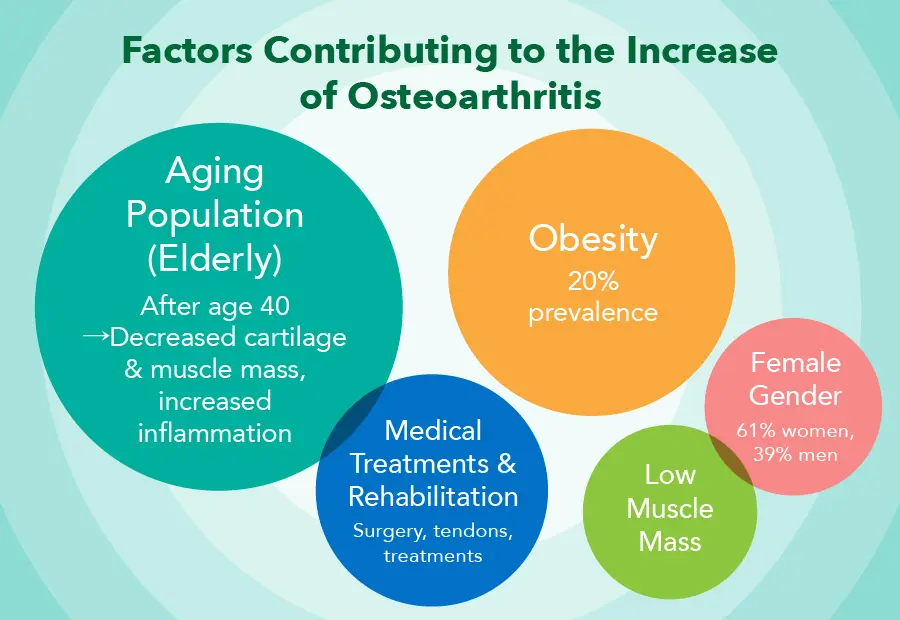
Causes of Osteoarthritis
Downward Spiral

Coming soon
LytoJoint™
In vivo Data
Experimental Data, Proving the Potential Benefit of LytoJoint™
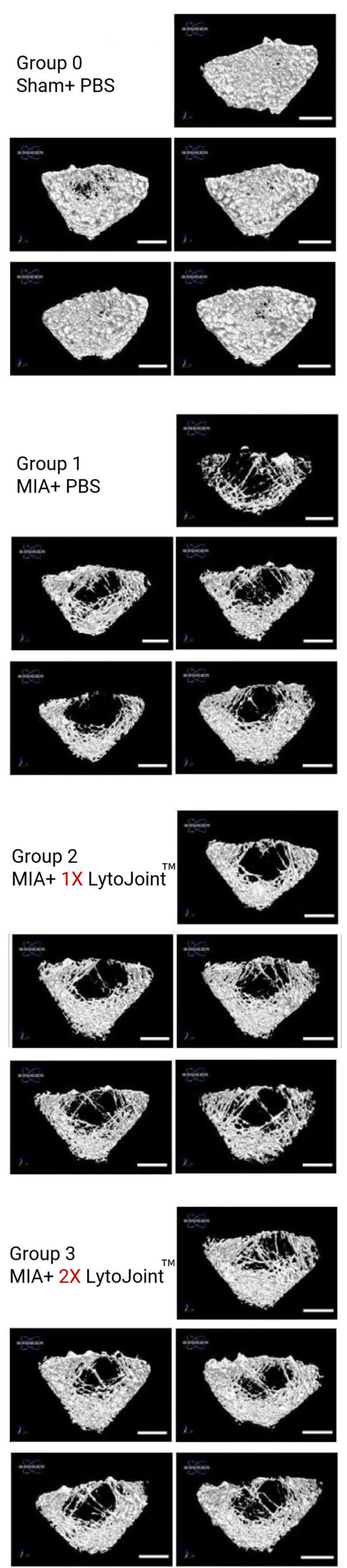
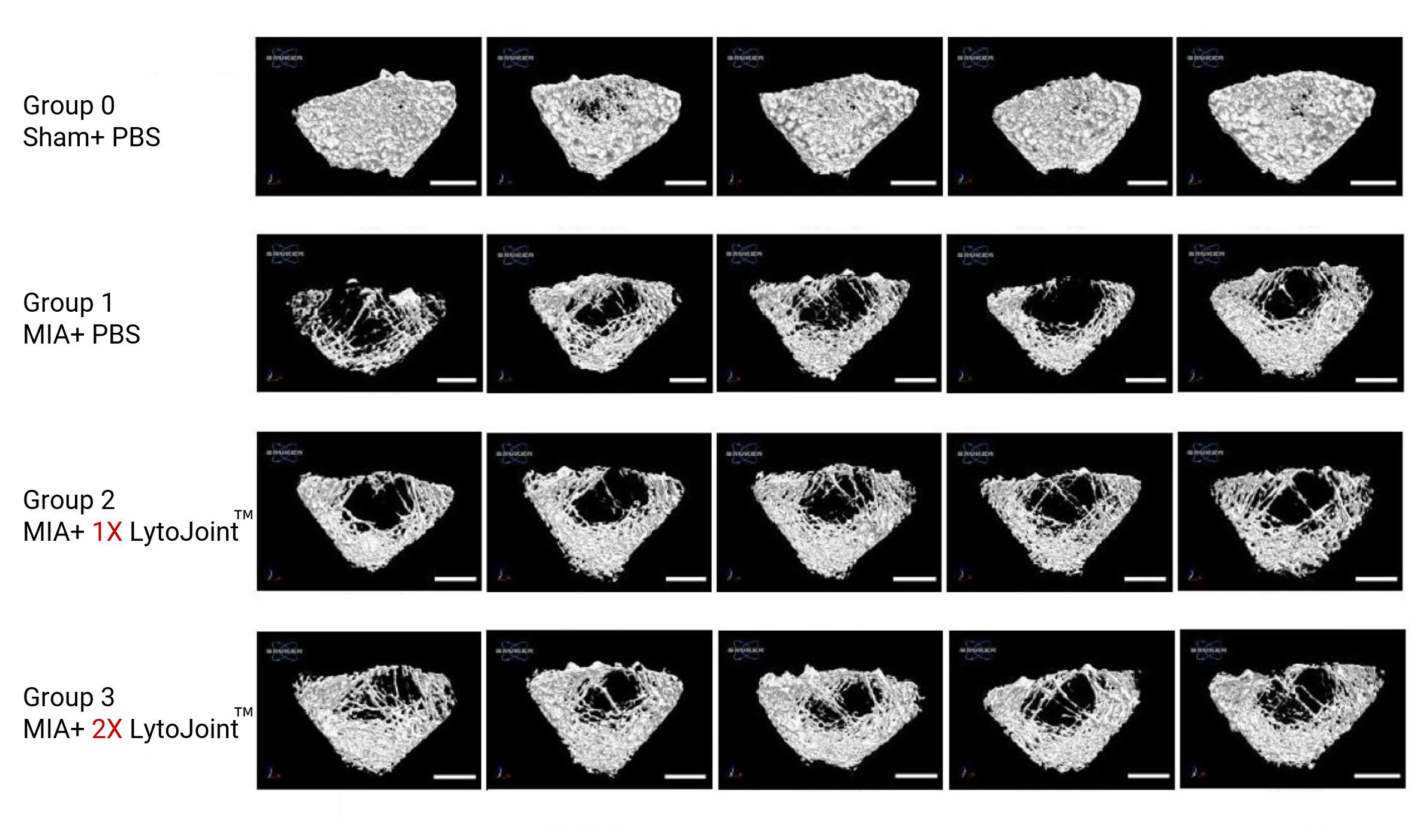
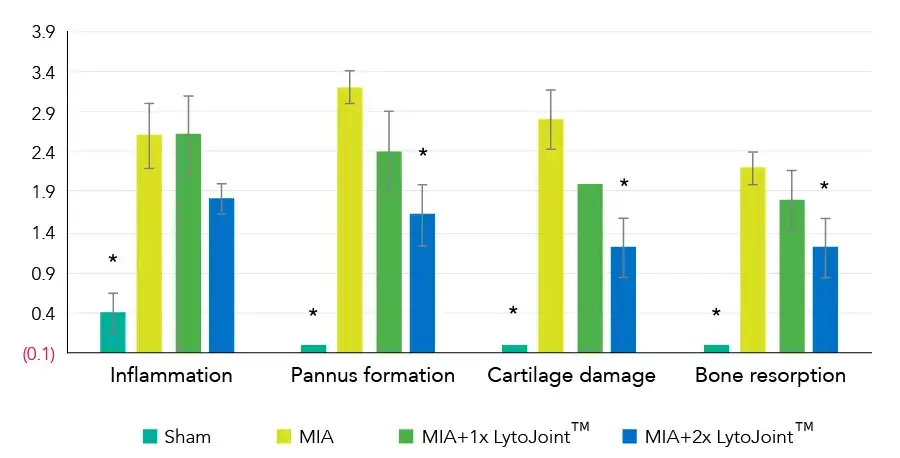
Total histological score
8-week-old female Sprague–Dawley rats (150–200 g, N = 20; BioLASCO Taiwan Ltd. Yilan, Taiwan) were maintained in cages under a 12-h light/12-h dark cycle with access to food and water ad libitum. This study was approved by the Institutional Animal Care and Use Committee (IACUC 2023-R403-055), and all experiments were conducted in accordance with the relevant guidelines.
MIA-induced OA model
To induce OA, MIA (4 mg in 50 µL of saline; Sigma-Aldrich, St. Louis, MO, USA) was injected into the right knee joint cavity of anesthetized rats (2–3% isoflurane in O2), using a 26G syringe.
Development Center for Biotechnology; Institute for Drug Evaluation Platform. Taiwan